Phosphorus

Explore the vibrant world of Phosphorus, an element that ignites curiosity and innovation in the scientific community. This guide is your pathway to understanding its intricate nature, practical uses, and why it’s often paired with Hydrogen in various reactions and applications. Dive into examples that illuminate its role in nature and technology, making your teachings about Phosphorus as radiant as the element itself. Perfect for educators looking to enrich their knowledge and spark student interest!
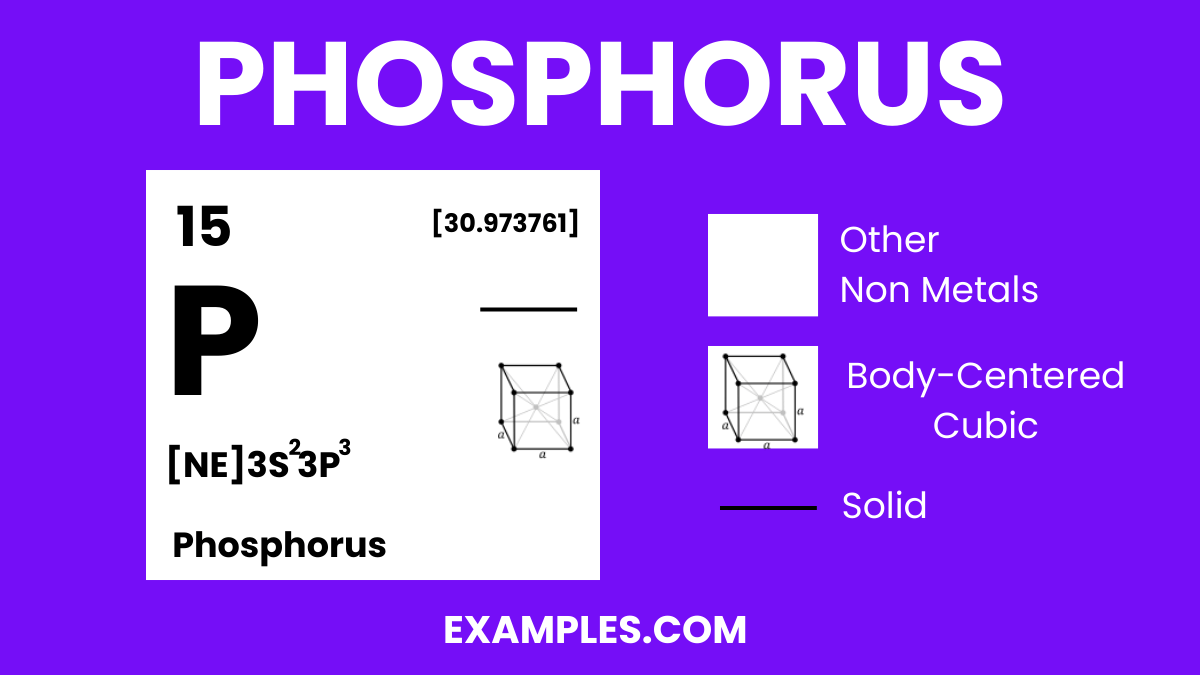
What is Phosphorus?
Phosphorus is a chemical element with the symbol P and atomic number 15. It is a non-metal that typically exists in two major forms—white and red phosphorus—each with distinct properties and uses. As an essential element in life, it plays a critical role in biological processes, including the formation of DNA, cell membranes, and bone structure. It’s also widely used in industry for creating fertilizers, safety matches, and other products. Understanding Phosphorus is key to comprehending various chemical reactions and natural phenomena.
Other Reactive Nonmetals
| Hydrogen | Sulfur |
| Carbon | Chlorine |
| Nitrogen | Selenium |
| Oxygen | Bromine |
| Fluorine | Iodine |
Phosphorus Formula
- Formula: P₄
- Composition: Four phosphorus atoms.
- Bond Type: Strong single bonds forming a tetrahedral structure.
- Molecular Structure: Tetrahedral.
- Electron Configuration: Five valence electrons per atom, twenty in total for P₄.
- Significance: Vital for life, integral in DNA and ATP, crucial for agriculture.
- Role in Chemistry: Forms various compounds used in fertilizers, detergents, and industrial processes. Exists mainly as white or red phosphorus with diverse applications.
Atomic Structure of Phosphorus

Physical Properties ofPhosphorus

ChemicalProperties ofPhosphorus
Phosphorus is known for its high reactivity, especially white phosphorus. It exhibits various chemical behaviors:
- Reactivity with Oxygen: Phosphorus readily reacts with oxygen in air, forming phosphorus pentoxide. 4P+5O₂→P₄O₁₀
- Reactivity with Halogens: It reacts with halogens to form phosphorus halides, such as phosphorus trichloride. 2P+3Cl₂→2PCl₃
- Reaction with Metals: Phosphorus can react with certain metals to form phosphides. 3Ca+2P→Ca₃P₂ (Calcium Phosphide)
- Acidic Oxides Production: The pentoxide form reacts with water to produce phosphoric acid, an important chemical in fertilizers and detergents. P₄O₁₀+6H₂O→4H₃PO₄
These properties make phosphorus a highly valuable and versatile element in various chemical processes, including fertilizers, safety matches, pesticides, and more. Its ability to react and form a wide range of compounds is fundamental to its extensive use in industrial and household applications.
Thermodynamic Properties of Phosphorus
| Property | Value with Unit |
|---|---|
| Boiling Point (white P) | 280.5 °C |
| Melting Point (white P) | 44.15 °C |
| Heat of Vaporization | 51.9 kJ/mol (white P) |
| Heat of Fusion | 2.51 kJ/mol (white P) |
| Specific Heat Capacity (white P, 25°C) | 0.769 J/g·K |
| Thermal Conductivity (white P) | 0.236 W/m·K |
Material Properties of Phosphorus
| Property | Value with Unit |
|---|---|
| Density (white P, 20°C) | 1.823 kg/m³ |
| Solubility in Water | Insoluble (white P) |
| Mohs Hardness (white P) | 0.5 |
| Crystal Structure (white P) | Cubic |
Electromagnetic Properties of Phosphorus
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (white P) | High (Insulator) |
| Electronegativity (Pauling scale) | 2.19 |
| Electron Affinity | 72 kJ/mol |
Nuclear Properties of Phosphorus
| Property | Value with Unit |
|---|---|
| Atomic Number | 15 |
| Atomic Mass | 30.973762 amu |
| Isotopes | ^31P (100%) |
| Nuclear Spin (for ^31P) | 1/2 ℏ |
| Neutron Cross Section (for ^31P) | 0.172 barns |
| Nuclear Magnetic Moment (for ^31P) | 1.1316 µN |
Allotropes of Phosphorus
Phosphorus is unique due to its several allotropes, mainly white, red, and black phosphorus, each with distinct physical and chemical properties. Understanding these allotropes is crucial as they determine the element’s reactivity and use in various applications.
White Phosphorus (P₄)
Appearance and Properties: White phosphorus consists of P₄ tetrahedra, where each atom is bonded to the other three. It’s waxy, white, and highly reactive, especially with oxygen. It must be stored underwater to prevent it from igniting in air.
Reactivity: White phosphorus is highly reactive and is used in incendiary devices and smoke screens. It can spontaneously ignite in air at around 30°C.
Equation Example: When white phosphorus reacts with chlorine, phosphorus trichloride is formed. P₄+6Cl₂→4PCl₃
Red Phosphorus
Appearance and Properties: Red phosphorus is more stable and safer to handle than white phosphorus. It’s an amorphous network that lacks the P₄ structure.
Uses: It’s used in safety matches, fireworks, and other pyrotechnics. Unlike white phosphorus, it doesn’t ignite in air and is less toxic.
Conversion from White Phosphorus: White phosphorus can be converted to red phosphorus by heating under controlled conditions.
Black Phosphorus
Appearance and Properties: Black phosphorus is the least reactive allotrope and has a layered structure, similar to graphite. It’s a good conductor of electricity.
Uses: Due to its unique electrical and mechanical properties, it’s researched for use in electronics, semiconductors, and batteries.
Formation: It’s obtained by heating white phosphorus under high pressures.
Understanding these allotropes allows for better utilization and handling of phosphorus in various industrial and scientific applications. Each allotrope’s unique properties cater to specific needs, making phosphorus a versatile and widely used element in the modern world. Whether it’s in the manufacturing of safety matches or the potential future of electronics with black phosphorus, this element’s varied forms are integral to numerous sectors.
Chemical Compounds of Phosphorus
Phosphorus forms a wide array of compounds, each with its unique properties and applications. The versatility of phosphorus in forming compounds lies in its ability to bond with many different elements, including oxygen, hydrogen, and various metals. Here’s an in-depth look at some of the most significant phosphorus compounds:
Phosphates (PO₄³⁻)
Phosphates are perhaps the most well-known compounds of phosphorus, essential in biochemistry, agriculture, and industry. They are formed by the combination of phosphoric acid with metals. One of the most common phosphates is calcium phosphate, used in fertilizers and as a nutritional supplement.
Equation: 3Ca(OH)₂+2H₃PO₄→Ca₃(PO₄)₂+6H₂O (Formation of Calcium Phosphate)
Phosphoric Acid (H₃PO₄)
Phosphoric acid is a weak acid that forms when phosphorus pentoxide reacts with water. It’s widely used in soft drinks, as a rust remover, and in fertilizers.
Equation: P4O₁₀+6H₂O→4H₃PO₄ (Formation of Phosphoric Acid)
Phosphorus Halides
Phosphorus forms a series of halides, the most common being phosphorus trichloride (PCl₃) and phosphorus pentachloride (PCl₅). These compounds are primarily used in organic synthesis and as chlorinating agents.
Equations: 2P+3Cl₂→2PCl₃ (Formation of Phosphorus Trichloride) P+5Cl₂→PCl₅(Formation of Phosphorus Pentachloride)
Phosphine (PH₃)
Phosphine is a toxic and flammable gas formed when calcium phosphide reacts with water or acid. It’s used as a fumigant and in the semiconductor industry.
Equation: Ca₃P₂+6H₂O→2PH₃+3Ca(OH)₂(Formation of Phosphine)
Organic Phosphorus Compounds
Organophosphates are widely used as insecticides and nerve agents. These compounds work by disrupting the nervous system’s function and are derived from phosphoric acid. A common example is glyphosate, a widely used herbicide.
Equation: C₃H₈NO₅P+H₂O→(Glyphosatehydrolysis)
By understanding these compounds and their reactions, one can appreciate the vital role of phosphorus in various fields, from agriculture and biochemistry to industrial applications. The unique chemical behavior of phosphorus allows it to form a wide array of compounds, each serving important functions in the world around us. As we continue to study and harness these compounds, phosphorus remains at the forefront of scientific and industrial innovation.
Isotopes of Phosphorus
Phosphorus has several isotopes, with Phosphorus-31 being the most stable and abundant in nature. Here’s a table describing some of the isotopes of Phosphorus:
| Isotope | Atomic Mass | Half-Life | Decay Mode | Natural Abundance |
|---|---|---|---|---|
| Phosphorus-31 | 30.97376199 | Stable | — | 100% |
| Phosphorus-32 | 31.97390727 | 14.28 days | Beta decay | Trace |
| Phosphorus-33 | 32.9717255 | 25.3 days | Beta decay | Trace |
Phosphorus-31 is the only stable isotope and has significant applications in nuclear magnetic resonance (NMR) due to its nuclear spin properties. Phosphorus-32 and Phosphorus-33 are radioactive and used in various scientific and medical applications, including tracers and treatment of specific types of cancer.
Uses of Phosphorus
Phosphorus is a versatile element with numerous applications across various fields. Here are some of the most significant uses of phosphorus:
- Fertilizers: The largest use of phosphorus is in the production of fertilizers. Phosphates, derived from phosphoric acid, are a primary nutrient for plant growth, making them an essential component in agriculture.
- Detergents: Phosphates are also used in detergents to soften water and enhance cleaning efficiency.
- Safety Matches: Red phosphorus is used on the striking surface of safety matches, as it ignites when struck against a rough surface.
- Steel Production: Phosphorus is used as an alloying agent in steel to improve strength and corrosion resistance.
- Pesticides: Certain phosphorus compounds are used to make pesticides, herbicides, and insecticides.
- Food Industry: Phosphates act as leavening agents in baking and are used in processed foods to improve texture and moisture retention.
- Flame Retardants: Certain phosphorus compounds are used as flame retardants in textiles, plastics, and electronics.
- Medicine: Radioactive isotopes of phosphorus are used in medical treatments, including chemotherapy for certain types of cancer.
- LEDs and Semiconductors: Phosphorus is used in the manufacturing of LEDs and semiconductors.
Biological and Physiological Significance of Phosphorus
Phosphorus plays a pivotal role in the biological and physiological functions of all living organisms. Here are the key points detailing its significance:
- Structural Component of DNA and RNA: Phosphorus forms part of the backbone of DNA and RNA molecules, which are crucial for genetic inheritance and protein synthesis.
- Energy Transfer: It is a constituent of adenosine triphosphate (ATP), the molecule used by cells for energy transfer and storage.
- Bone and Teeth Formation: Phosphorus, in the form of phosphate ions, combines with calcium to form the calcium phosphate that gives strength and structure to bones and teeth.
- Cell Membrane Integrity: Phospholipids, which contain phosphorus, are fundamental components of cell membranes, ensuring cell integrity and facilitating communication between cells.
- Enzyme Activation: It is involved in the activation of many enzymes and hormones by phosphorylation, thus playing a critical role in the regulation of cellular processes.
- Buffer System: Phosphorus is a part of the body’s buffer system, helping maintain pH balance in blood and other fluids.
- Metabolic Pathways: It is involved in several key metabolic pathways, including those that convert food into energy.
By understanding these roles, it’s evident that phosphorus is not merely an element on the periodic table but a cornerstone of life and vitality.
Commercial Production of Phosphorus
The commercial production of phosphorus primarily involves the extraction and processing of phosphate rock. The process is optimized for efficiency, environmental impact, and quality of the final product. Here’s how phosphorus is commercially produced:
- Mining of Phosphate Rock: The first step is the extraction of phosphate rock, containing phosphorus in the form of phosphate minerals.
- Beneficiation: The extracted ore undergoes beneficiation, a series of processes including washing, screening, and sometimes flotation, to increase the phosphorus concentration and remove impurities.
- Thermal Process: The most common method for producing phosphorus is through a thermal process where phosphate rock is heated with co*ke and silica in an electric arc furnace. This process produces phosphorus gas and slag. 2Ca3(PO4)2+6SiO2+10C→6CaSiO3+10CO+P4
- Condensation: The phosphorus gas is then condensed into a liquid or solid form under controlled conditions.
- Purification: The collected phosphorus may undergo further purification to achieve the desired quality, depending on its intended use.
- Forming and Packaging: Finally, phosphorus is formed into specific shapes or combined with other elements to produce compounds and then packaged for commercial use.
In commercial settings, phosphorus is primarily used to produce phosphoric acid, a precursor to fertilizers, and various other phosphorus compounds that serve as flame retardants, pesticides, and in numerous other applications. The production is continuously optimized for higher yields, better quality, and reduced environmental impact, reflecting the critical nature of this element in a wide array of industrial and agricultural processes.
Health Effects of Phosphorus
Phosphorus is a vital element in the human body and plays several key roles in maintaining health and well-being. However, both deficiency and excess of phosphorus can lead to health issues:
- Bone and Teeth Strength: Phosphorus is crucial for the formation and maintenance of bones and teeth. It works in tandem with calcium to enhance bone density and structure.
- Energy Production: As part of ATP (adenosine triphosphate), phosphorus is vital for storing and transferring energy within cells, supporting metabolism and physical activity.
- Deficiency Concerns: Phosphorus deficiency, though rare, can lead to weakened bones, joint pain, fatigue, irregular breathing, and irritability. It’s usually seen in cases of severe malnutrition or certain medical conditions.
- Toxicity and Overexposure: Excessive intake of phosphorus, especially from dietary supplements or certain medications, can disrupt calcium balance, leading to bone loss and calcification of organs and tissues.
- Kidney Function: High levels of phosphorus can strain kidney functions, particularly in those with existing kidney conditions. It may lead to the formation of kidney stones and other renal problems.
- Muscle and Nerve Function: Adequate phosphorus levels are necessary for normal muscle contraction and nerve signaling, impacting overall muscle health and neurological function.
Environmental Effects of Phosphorus
Phosphorus, while essential for life, can have profound environmental impacts, especially when its natural balance is disrupted:
- Eutrophication: Excess phosphorus in water bodies, often due to agricultural runoff containing fertilizers, can lead to eutrophication. This process results in excessive growth of algae and depletion of oxygen, harming aquatic life and degrading water quality.
- Soil Fertility: Phosphorus is a key nutrient in soil, promoting plant growth. However, both deficiency and surplus can affect soil health and productivity. Proper management is necessary to maintain soil vitality.
- Water Quality: High levels of phosphorus in water can lead to contamination, making it unsafe for drinking and harming aquatic ecosystems. It’s crucial to monitor and control phosphorus levels in water bodies.
- Biodiversity: Changes in phosphorus levels can alter ecosystems, affecting biodiversity. Plants and animals that can’t adapt to these changes may decline or disappear, leading to reduced ecological variety.
- Algal Blooms: One of the most visible effects of phosphorus pollution is algal blooms, which can produce toxins harmful to wildlife and humans. These blooms can disrupt recreational activities and water treatment processes.
What is the Chemical Name for Phosphorus?
The chemical name for phosphorus is simply “Phosphorus” with the chemical symbol P and atomic number 15.
What is the Use of Phosphorus?
Phosphorus is used in fertilizers, detergents, pesticides, and is vital for DNA, bones, and energy transfer in living organisms.
Is Phosphorus P4 or P?
Phosphorus exists mainly as P4 in its elemental form, especially as white phosphorus, which consists of P4 tetrahedra.
Where is Phosphorus From?
Phosphorus is primarily obtained from phosphate rocks and is widely distributed in the earth’s crust and living organisms.
Why is Phosphorus a Critical Element?
Phosphorus is critical for life, involved in DNA, energy transfer, bone health, and essential in agriculture and industry.
Phosphorus is an essential element, intricately woven into the tapestry of life and industry. Understanding its properties, uses, and impact is crucial for students, educators, and professionals. By mastering how to write and convey its importance, one can appreciate Phosphorus’s role in the natural and scientific world, leading to more informed, sustainable decisions and innovative applications.
